Details of the Drug
General Information of Drug (ID: DMHAWYQ)
| Drug Name |
Nordazepam
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Calmday; Chlordesmethyldiazepam; Dealkylprazepam; Demadar; Demethyldiazepam; Desalkylprazepam; N-Demethyldiazepam; N-Deoxydemoxapam; N-Deoxydemoxepam; N-Descyclopropylmethylprazepam; N-Desmethyldiazepam; N1-Desmethyldiazepam; Nordaz; Nordazepam; Nordazepam [INN]; Nordazepamum; Nordazepamum [INN-Latin]; Nordiazepam; Norprazepam; Praxadium; Ro 5-2180; Stilny; Vegesan; desmethyldiazepam; 1-Demethyldiazepam; 1088-11-5; 7-Chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one; A 101; A101; DMDZ; Lomax; Madar; NSC 46078; Sopax
|
|||||
| ATC Code | ||||||
| Structure |
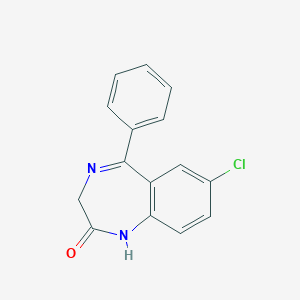 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 270.71 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | |||||
| Rotatable Bond Count (rotbonds) | 1 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
