Details of the Drug
General Information of Drug (ID: DMHKCIS)
| Drug Name |
Aldoxorubicin
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1361644-26-9; ALDOXORUBICIN; Doxorubicin-EMCH; 151038-96-9; Aldoxorubicin (USAN); CHEMBL2107818; SCHEMBL15221892; OBMJQRLIQQTJLR-LBMCFUDOSA-N; ZINC163337436; AKOS030526452; CS-1186; HY-16261; D10383; W-5595; (8S)-1-Methoxy-6,8alpha,11-trihydroxy-8-[1-[2-[6-(2,5-dioxo-3-pyrroline-1-yl)hexanoyl]hydrazono]-2-hydroxyethyl]-10alpha-(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyloxy)-7,8,9,10-tetrahydronaphthacene-5,12-dione
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Structure |
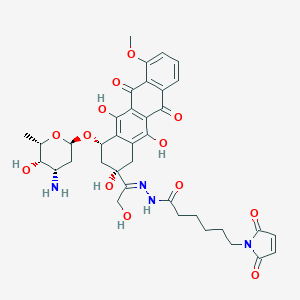 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 750.7 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 12 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
