Details of the Drug
General Information of Drug (ID: DMHN7CB)
| Drug Name |
Tributylstannanyl
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tributyltin; tributylstannanyl; Tributlytin; Tributylstannic hydride; TRIBUTYL TIN; Tri-n-butylstannane hydride; UNII-4XDX163P3D; Tin, tri-n-butyl-, hydride; HSDB 6362; EINECS 211-704-4; BRN 3587329; 4XDX163P3D; Stannane, tri-n-butyl-, hydride; 20763-88-6; Tri-n-butyltin hydride, 97%; tri-n-butylstannyl radical; tributyl-tin; TBY; TBTH; AC1MHUMB; AC1Q2UP8; ACMC-1B91A; DTXSID0040709; CTK1A6693; MolPort-003-928-307; PIILXFBHQILWPS-UHFFFAOYSA-N; MolPort-035-869-643; EBD10558; ANW-35557; MFCD00009416; ZINC169748512; AKOS015909735
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
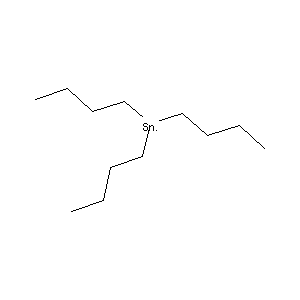 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 290.05 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 0 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
