| Drug Name |
MDL-105519
|
| Synonyms |
Mdl 105519; MDL 105,519; 161230-88-2; Lopac-M-216; AC1O7G4O; Lopac0_000805; C18H11Cl2NO4; SCHEMBL499902; CHEMBL180427; AOB5684; HMS3262B11; ZINC1540415; Tox21_500805; AKOS027324667; LP00805; CCG-204889; CS-3882; NCGC00015639-04; NCGC00015639-01; NCGC00094138-02; NCGC00015639-03; NCGC00261490-01; NCGC00015639-02; HY-15085; X6860; M-216; EU-0100805; SR-01000075460; SR-01000075460-1
|
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
376.2 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.8 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
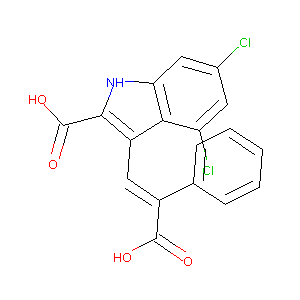
- Formula
- C18H11Cl2NO4
- IUPAC Name
3-[(E)-2-carboxy-2-phenylethenyl]-4,6-dichloro-1H-indole-2-carboxylic acid - Canonical SMILES
-
C1=CC=C(C=C1)/C(=C\\C2=C(NC3=C2C(=CC(=C3)Cl)Cl)C(=O)O)/C(=O)O
- InChI
-
InChI=1S/C18H11Cl2NO4/c19-10-6-13(20)15-12(16(18(24)25)21-14(15)7-10)8-11(17(22)23)9-4-2-1-3-5-9/h1-8,21H,(H,22,23)(H,24,25)/b11-8+
- InChIKey
-
LPWVUDLZUVBQGP-DHZHZOJOSA-N
|
| Cross-matching ID |
- PubChem CID
- 6603913
- TTD ID
- D0X5PX
|
|
|
|
|
|
|
|


