Details of the Drug
General Information of Drug (ID: DMHVEGJ)
| Drug Name |
CC-930
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TANZISERTIB; CC-930; 899805-25-5; CC 930; UNII-M5O06306UO; CHEMBL1950289; M5O06306UO; CC930; Trans-4-({9-[(3s)-Tetrahydrofuran-3-Yl]-8-[(2,4,6-Trifluorophenyl)amino]-9h-Purin-2-Yl}amino)cyclohexanol; Tanzisertib [USAN:INN]; 3tti; trans-4-((9-((3S)-Tetrahydrofuran-3-yl)-8-((2,4,6-trifluorophenyl)amino)-9H-purin-2-yl)amino)cyclohexanol; Tanzisertib (USAN); CC-930(Tanzisertib); SCHEMBL2133061; SCHEMBL4760419; SCHEMBL2133055; GTPL9836; SCHEMBL8082102; SCHEMBL15589918; CHEMBL1950305; SCHEMBL10179476; JNK-930; MolPort-044-562-358
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
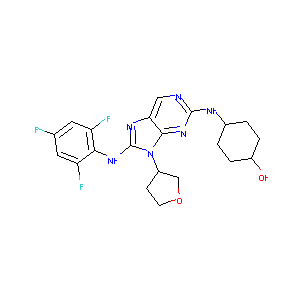 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 448.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
