Details of the Drug
General Information of Drug (ID: DMHZBEO)
| Drug Name |
Difelikefalin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-NA1U919MRO; NA1U919MRO; MR13A9; 1024828-77-0; FE202845; Difelikefalin [INN]; Difelikefalin [USAN:INN]; SEQ ID NO: 2; GTPL9044; CHEMBL3989915; SCHEMBL10316464; BDBM235785; DB11938; FE-202845; US9359399, 2; D-Phe-D-Phe-D-Leu-D-Lys-[gamma-(4-N-piperidinyl)amino carboxylic acid]; 4-Piperidinecarboylic acid, 4-amino-1-(D-phenylalanyl-D-phenylalanyl-D-leucyl-D-lysyl)-; 4-Piperidinecarboxylic acid, N1-(D-phenylalanyl-D-phenylalanyl-D-leucyl-D-lysyl)-4-amino-; 4-Piperidinecarboxylic acid, N1-(D
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
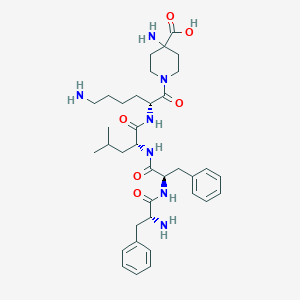 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 679.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 18 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pruritus | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EC90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
