Details of the Drug
General Information of Drug (ID: DMI3E0Y)
| Drug Name |
MK6-83
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1062271-24-2; UNII-RE9JUR6NT4; RE9JUR6NT4; 5-Methyl-N-[2-(1-piperidinyl)phenyl]-2-thiophenesulfonamide; C16H20N2O2S2; 5-methyl-N-(2-(piperidin-1-yl)phenyl)thiophene-2-sulfonamide; 5-methyl-N-(2-piperidin-1-ylphenyl)thiophene-2-sulfonamide; 5-Methyl-N-(2-(1-piperidinyl)phenyl)-2-thiophenesulfonamide; GTPL9783; AOB6422; SYN5108; ZINC69572886; AKOS025147398; NCGC00402264-02; NCGC00402264-04; AS-16472; CID 18191179; HY-110238; CS-0033102; 2-Thiophenesulfonamide, 5-methyl-N-(2-(1-piperidinyl)phenyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
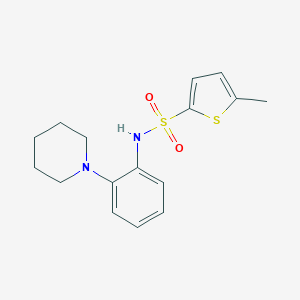 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 336.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
