Details of the Drug
General Information of Drug (ID: DMIFYLX)
| Drug Name |
Bopindolol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BOPINDOLOL; 62658-63-3; Sandonorm; (+-)-Bopindolol; 1-(tert-Butylamino)-3-((2-methyl-1H-indol-4-yl)oxy)propan-2-yl benzoate; Bopindololum [INN-Latin]; Bopindolol [INN]; UUOJIACWOAYWEZ-UHFFFAOYSA-N; Bopindolol (INN); Wandonorm; 69010-88-4; NCGC00163155-01; Sandonorm (TN); (+-)-4-(2-Benzoyloxy-3-tert-butylaminopropoxy)-2-methylindole; DSSTox_CID_2684; DSSTox_RID_76690; (+-)-1-(tert-Butylamino)-3-((2-methylindol-4-yl)oxy)-2-propanol benzoate (ester); DSSTox_GSID_22684
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
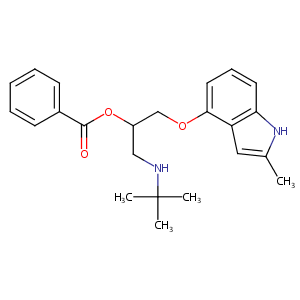 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 380.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
