Details of the Drug
General Information of Drug (ID: DMIGX84)
| Drug Name |
Cethromycin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Restanza; Cethromycin [USAN]; ABT 773; A-195773; ABT-773; Abbott-195773; A-195773.0; Cethromycin (USAN/INN); (1R,2R,4R,6R,7R,8R,10R,13R,14S)-7-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-13-ethyl-2,4,6,8,10,14-hexamethyl-6-[(E)-3-quinolin-3-ylprop-2-enoxy]-12,15-dioxa-17-azabicyclo[12.3.0]heptadecane-3,9,11,16-tetrone; (1R,2R,4R,6S,7R,8R,10R,13R,14S)-7-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-13-ethyl-2,4,6,8,10,14-hexamethyl-6-[(E)-3-quinolin-3-ylprop-2-enoxy]-12,15-dioxa-17-azabicyclo[12.3.0]heptadecane-3,9,11,16-tetrone; (1S,2R,5R,7R,8R,9R,11R,13R,14R)-2-Ethyl-1,5,7,9,11,13-Hexamethyl-9-[3-(3-quinolyl)-2(E)-propenyloxy]-8-[3,4,6-trideoxy-3-(dimethylamino)-beta-D-glucopyranosyloxy]-3,17-dioxa-15-azabicyclo[12.3.0]hepta; (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-Ethyl-3a,7,9,11,13,15 hexamethyl-11-((3-(quinolin-3-yl)prop-2-enyl)oxy)-10-((3,4,6-trideoxy-3-(dimethylamino)-beta-D-xylo-hexopyranosyl)oxy)octahydro-2H-oxacyclotetradecino(4,3-d)oxazole-2,6,8,14(1H,7H,9H)-tetrone; (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-Ethyl-3a,7,9,11,13,15-hexamethyl-11-((3-(quinolin-3-yl)prop-2-enyl)oxy)-10-((3,4,6-trideoxy-3-(dimethylamino)-beta-D-xylo-hexopyranosyl)oxy)octahydro-2H-oxacyclotetradecino(4,3-d)oxazole-2,6,8,14(1H,7H,9H)-tetrone
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Bacteria
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
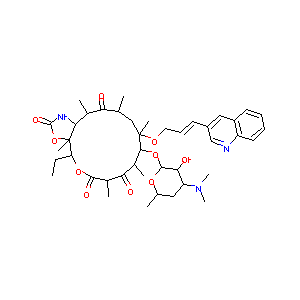 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 765.9 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
