Details of the Drug
General Information of Drug (ID: DMJ6PB8)
| Drug Name |
ETHYLGALLATE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ETHYL GALLATE; 831-61-8; Ethyl 3,4,5-trihydroxybenzoate; Gallic acid ethyl ester; Phyllemblin; Nipagallin A; Progallin A; Ethylgallate; Nipa No. 48; Gallic acid, ethyl ester; Benzoic acid, 3,4,5-trihydroxy-, ethyl ester; NIPA 48; 3,4,5-Trihydroxybenzoic acid ethyl ester; Ethyl-3,4,5-trihydroxybenzoate; Ethylester kyseliny gallove; UNII-235I6UDD3L; NSC 402626; Ethylester kyseliny gallove [Czech]; EINECS 212-608-5; BRN 2116014; CHEMBL453196; 235I6UDD3L; CHEBI:87247; VFPFQHQNJCMNBZ-UHFFFAOYSA-N; NSC402626; AK-94174; Q-100846; Gallic aci
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
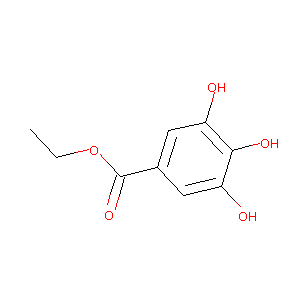 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 198.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
