Details of the Drug
General Information of Drug (ID: DMJ816F)
| Drug Name |
Sodelglitazar
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sodelglitazar; 447406-78-2; UNII-6G973E04VI; 6G973E04VI; GW677954; Sodelglitazar [USAN:INN]; 2-[4-[[[2-[2-Fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methyl]sulfanyl]-2-methylphenoxy]-2-methylpropanoic acid; 2-(4-(((2-(2-Fluoro-4-(trifluoromethyl)phenyl)-4-methyl-1,3-thiazol-5-yl)methyl)sulfanyl)-2-methylphenoxy)-2-methylpropanoic acid; Sodelglitazar (USAN); SCHEMBL4822839; CHEMBL2104984; DTXSID90196289; ZUGQWAYOWCBWGM-UHFFFAOYSA-N; ZINC1553281; AN-28238; ACM447406782; FT-0743554; D06647; 406S782; Propanoic ac
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
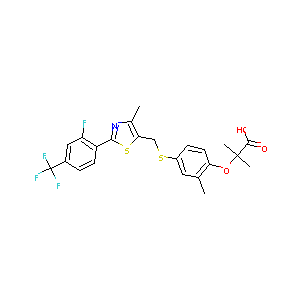 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 499.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperlipidaemia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5C80 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
