| Drug Name |
Hydroxyamphetamine
|
| Synonyms |
Hydroxyamfetamine; Hydroxyamphetamide; Norpholedrine; Norveritol; Nov-Pholedrin; Oksamfetamin; Oxamfetamin; Oxamphetamine; Oxamphetaminium; Paradrine; Paredrine; Paredrinex; Pedrolon; Phenol, 4-(2-aminopropyl)-; Phenol, p-(2-aminopropyl)-; Phenol,4-(2-aminopropyl)-; Pulsoton; alpha-Methyltyramine; hydroxyamphetamine; p-(2-Aminopropyl)phenol; p-HYDROXYAMPHETAMINE; .alpha.-Methyltyramine; 1-p-Hydroxyphenyl-2-propylamine; 103-86-6; 2-Amino-1-(p-hydroxyphenyl)propane; 4-(2-Aminopropyl)phenol; 4-Hydroxyamphetamine
|
| Structure |
|
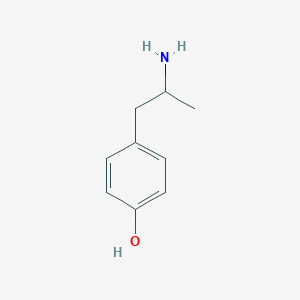 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
151.21 |
|
| Logarithm of the Partition Coefficient (xlogp) |
1.4 |
| Rotatable Bond Count (rotbonds) |
2 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
2 |
| ADMET Property |
- Metabolism
-
The drug is metabolized via ophthalmic
[]
|
| Chemical Identifiers |
- Formula
- C9H13NO
- IUPAC Name
4-(2-aminopropyl)phenol - Canonical SMILES
-
CC(CC1=CC=C(C=C1)O)N
- InChI
-
GIKNHHRFLCDOEU-UHFFFAOYSA-N
- InChIKey
-
1S/C9H13NO/c1-7(10)6-8-2-4-9(11)5-3-8/h2-5,7,11H,6,10H2,1H3
|
| Cross-matching ID |
- PubChem CID
- 3651
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR1770
|
|
|
|
|
|
|
|

