Details of the Drug
General Information of Drug (ID: DMJDV0K)
| Drug Name |
SPR720
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-1NL76YUU6E; 1NL76YUU6E; CHEMBL2221212; VXC-486 phosphate; SCHEMBL10323399; BDBM50112815; ZINC95566061; HY-135655A; CS-0127906; 1384984-31-9; 2-[5-[2-(ethylcarbamoylamino)-6-fluoro-7-[(2R)-oxolan-2-yl]-3H-benzimidazol-5-yl]pyrimidin-2-yl]propan-2-yl dihydrogen phosphate; N-Ethyl-N'-(6-fluoro-5-(2-(1-methyl-1-(phosphonooxy)ethyl)-5-pyrimidinyl)-7-((2R)-tetrahydro-2-furanyl)-1H-benzimidazol-2-yl)urea; Urea, N-ethyl-N'-(6-fluoro-5-(2-(1-methyl-1-(phosphonooxy)ethyl)-5-pyrimidinyl)-7-((2R)-tetrahydro-2-furanyl)-1H-benzimidazol-2-yl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
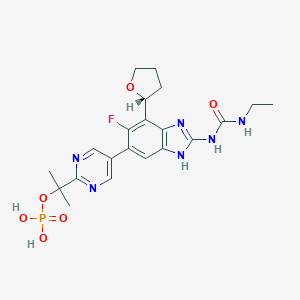 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 508.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
