Details of the Drug Combinations
General Information of This Drug (ID: DMJPV5X)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alovudine; 25526-93-6; FddT; 3'-DEOXY-3'-FLUOROTHYMIDINE; FddThD; 3'-Fluoro-3'-deoxythymidine; Thymidine, 3'-deoxy-3'-fluoro-; 3'F-TdR; 3'-FddT; Alovudine [USAN:INN]; UNII-PG53R0DWDQ; 3'-Fluorothymidine; 3'-FLT; DRG-0097; PG53R0DWDQ; NSC 140025; BRN 0754299; 3'-Fluorodeoxythymidine; CL 184824; CHEMBL105318; FLT; 1-((2R,4S,5R)-4-fluoro-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; C10H13FN2O4; MIV-310; 1-(3'-Deoxy-3'-fluoro-beta-D-pentofuranosyl)thymine; CL-184824; DSSTox_RID_81738; DSSTox_CID_26579
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
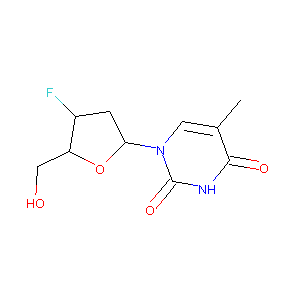 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
