| Drug Name |
Lorpiprazole
|
| Synonyms |
Lorpiprazole; Lorpiprazole [INN]; (-)-cis-5,5a,6,7,8,8a-Hexahydro-3-(2-(4-(alpha,alpha,alpha-trifluoro-m-tolyl)-1-piperazinyl)ethyl)cyclopenta(3,4)pyrrolo(2,1-c)-s-triazole; 108785-69-9; AC1MIILX; DTXSID30883181; UNII-0M14O7T47Q component BNRMWKUVWLKDQJ-CRAIPNDOSA-N
|
| Affected Organisms |
Humans and other mammals
|
| Structure |
|
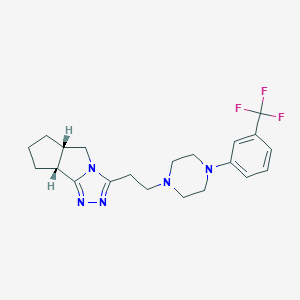 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
405.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.3 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| ADMET Property |
- Absorption Tmax
-
The time to maximum plasma concentration (Tmax) is 1 h
[]
- Half-life
-
The concentration or amount of drug in body reduced by one-half in 11 - 23 hours
[1]
- Metabolism
-
The drug is metabolized via the liver
[2]
|
| Chemical Identifiers |
- Formula
- C21H26F3N5
- IUPAC Name
(1R,8S)-5-[2-[4-[3-(trifluoromethyl)phenyl]piperazin-1-yl]ethyl]-3,4,6-triazatricyclo[6.3.0.02,6]undeca-2,4-diene - Canonical SMILES
-
C1CC2CN3C(=NN=C3C2C1)CCN4CCN(CC4)C5=CC=CC(=C5)C(F)(F)F
- InChI
-
BNRMWKUVWLKDQJ-CRAIPNDOSA-N
- InChIKey
-
1S/C21H26F3N5/c22-21(23,24)16-4-2-5-17(13-16)28-11-9-27(10-12-28)8-7-19-25-26-20-18-6-1-3-15(18)14-29(19)20/h2,4-5,13,15,18H,1,3,6-12,14H2/t15-,18-/m1/s1
|
| Cross-matching ID |
- PubChem CID
- 3045380
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR0982
|
|
|
|
|
|
|
|

