Details of the Drug
General Information of Drug (ID: DMKEGI3)
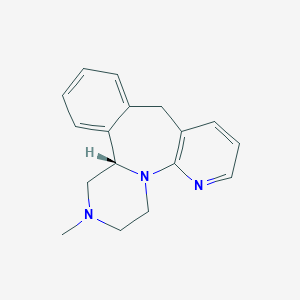
| Drug Name |
Esmirtazapine
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Esmirtazapine; Esmirtazapine [INN]; Org 44-20; Org-44-20; ZINC968310; (+)-Mirtazapine; (S)-1,2,3,4,10,14b-Hexahydro-2-methylpyrazino(2,1-a)pyrido(2,3-c)(2)benzazepine; 4685R51V7M; 61337-87-9; AC1MJ43F; BIDD:PXR0200; CHEMBL1366933; DB06678; EINECS 262-714-0; PDSP1_001530; PDSP2_001514; SCHEMBL49330; UNII-4685R51V7M
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.35 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | |||||
| Rotatable Bond Count (rotbonds) | 0 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
