Details of the Drug
General Information of Drug (ID: DMKJGTH)
| Drug Name |
1,8-bis-maleimidodiethyleneglycol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
115597-84-7; UNII-R20U86RE9F; 1,8-bis-maleimidodiethyleneglycol; 1,8-Bis(maleimido)-3,6-dioxaoctane; CHEMBL575383; R20U86RE9F; 2,2'-(Ethylenedioxy)bis(ethylmaleimide); Liqwd; 1H-Pyrrole-2,5-dione, 1,1'-(1,2-ethanediylbis(oxy-2,1-ethanediyl))bis-; Bis-(maleimidoethoxy) ethane; BM(peg)2; 1,1'-[ethane-1,2-Diylbis(Oxyethane-2,1-Diyl)]bis(1h-Pyrrole-2,5-Dione); 1H-Pyrrole-2,5-dione, 1,1'-[1,2-ethanediylbis(oxy-2,1-ethanediyl)]bis-; 1,2-bis(2-maleimidoethoxy)ethane; Bis-(maleimidoethoxy) ethane [INCI]; AC1MVX1G; SCHEMBL349749
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
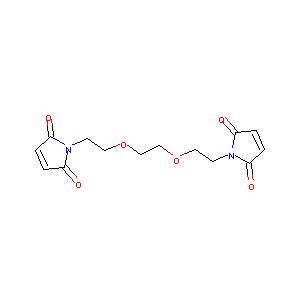 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 308.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
