Details of the Drug
General Information of Drug (ID: DMKQ0CA)
| Drug Name |
bromoenol lactone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bromoenol lactone; HELSS; 88070-98-8; 6-Btnpo; BTNP; C16H13BrO2; CHEMBL6206; BEL; 6-(Bromomethylene)tetrahydro-3-(1-naphthaleneyl)-2H-pyran-2-one; Haloenol lactone suicide substrate; BYUCSFWXCMTYOI-ZRDIBKRKSA-N; (E)-6-(Bromomethylene)tetrahydro-3-(1-naphthalenyl)-2H-Pyran-2-one; (E)-6-(Bromomethylene)-3-(1-naphthalenyl)tetrahydro-2H-pyran-2-one; bromoenolactone; E-6-(Bromoethylene)tetrahydro-3-(1-naphthyl)-2H-pyran-2-one; 2H-Pyran-2-one, 6-(bromomethylene)tetrahydro-3-(1-naphthalenyl)-, (E)-; SR-01000075707; bromoenolactone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
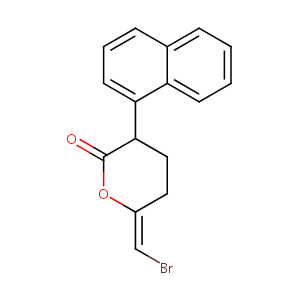 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 317.18 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
