Details of the Drug
General Information of Drug (ID: DMKTB5U)
| Drug Name |
PYRAZOLOPYRIDAZINE 1
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PYRAZOLOPYRIDAZINE 1; 551920-54-8; GW810576X; n-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-2-pyrimidinamine; pyrazolo[1,5-b]pyridazine deriv. 19; AC1O6ZIQ; CHEMBL187081; BDBM8128; SCHEMBL4489357; CTK1F7320; DTXSID60424889; HMS3305F24; HMS3303K24; ZINC13582569; NCGC00242229-01; DA-42106; FT-0707969; AB01092291-01; 2-Pyrimidinamine, N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-; N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
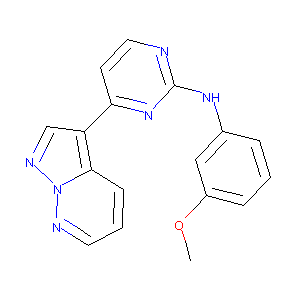 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 318.33 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
