Details of the Drug
General Information of Drug (ID: DML27AE)
| Drug Name |
Arverapamil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arverapamil; (R)-norverapamil; Agi-003; UNII-3J8P56R04P; (R)-(+)-Nor Verapamil Hydrochloride; 123932-43-4; 3J8P56R04P; Rezular; (+)-norverapamil; (R)-desmethylverapamil; (+)-desmethylverapamil; CHEBI:134082; ZINC13492624; AKOS030532539; Benzeneacetonitrile, alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)amino)propyl)-3,4-dimethoxy-alpha-(1-methylethyl)-, (alphaR)-; UNII-957Z3K3R56 component UPKQNCPKPOLASS-AREMUKBSSA-N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
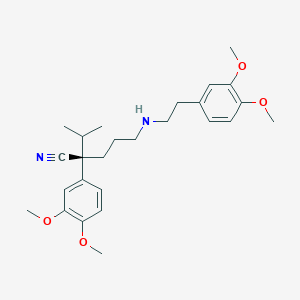 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 440.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
