Details of the Drug
General Information of Drug (ID: DMLKUG7)
| Drug Name |
Beclabuvir
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Beclabuvir; Beclabuvir (USAN/INN); Beclabuvir(BMS-791325); SB16521; 958002-33-0; BDBM50448498; BMS-791325; CHEMBL3126842; CS-6041; HY-12429; SCHEMBL11951525; cyclohexyl-N-(dimethylsulfamoyl)-methoxy-(3-methyl-3,8-diazabicyclo[3.2.1]octane-8-carbonyl)[?]carboxamide
|
|||||
| Structure |
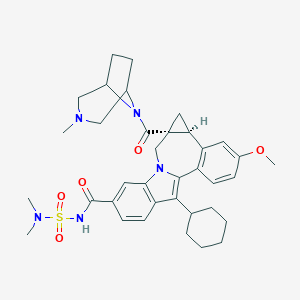 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 659.8 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | |||||
| Rotatable Bond Count (rotbonds) | 6 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
