Details of the Drug
General Information of Drug (ID: DMLQ3PO)
| Drug Name |
INCB13739
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Phenylarsine oxide; phenylarsine oxide; Oxophenylarsine; 637-03-6; Arzene; Phenylarsenoxide; Arsenosobenzene; ARSINE, OXOPHENYL-; Phenyl arsine oxide; Benzene, arsenoso-; arsorosobenzene; Fenylarsinoxid; Caswell No 060; Fenylarsinoxid [Czech]; C6H5AsO; Phenyl arsenoxide; UNII-0HUR2WY345; PAO; EINECS 211-275-3; NSC 42470; EPA Pesticide Chemical Code 007101; BRN 2935227; 0HUR2WY345; CHEBI:75253; MFCD00001990; Phenylarsine oxide, 97%; Phenylarsine Oxide Solution; oxo(phenyl)arsan; Phenylarsinoxyd; oxo(phenyl)arsine; oxo(phenyl)arsane; PhAsO; phenylarsenious
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
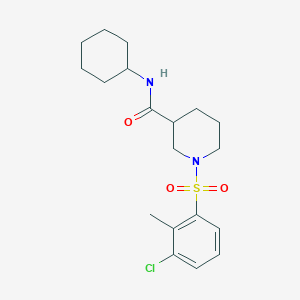 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 398.9 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Type-2 diabetes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A11 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
