Details of the Drug
General Information of Drug (ID: DMLQO7F)
| Drug Name |
EVP-6124
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Encenicline; EVP-6124; 550999-75-2; UNII-5FI5376A0X; EVP6124; EVP 6124; CHEMBL2151572; (r)-7-chloro-n-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide; 5FI5376A0X; C16H17ClN2OS; 550999-74-1; Encenicline [USAN:INN]; FRM-6124; Encenicline (USAN/INN); SCHEMBL744767; GTPL6926; SSRDSYXGYPJKRR-ZDUSSCGKSA-N; ZINC95579362; BDBM50393255; 3662AH; AKOS027322165; DB11726; CS-0933; MT-4666; Benzo(b)thiophene-2-carboxamide, N-(3R)-1-azabicyclo(2.2.2)oct-3-yl-7-chloro-; NCGC00378871-01; HY-15430; W-5978; D10626
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
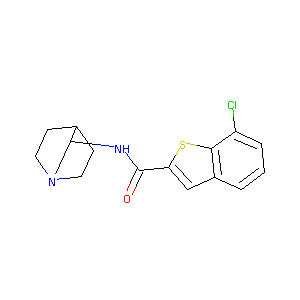 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 320.8 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
