Details of the Drug
General Information of Drug (ID: DMM9K5S)
| Drug Name |
Cmp-2-Keto-3-Deoxy-Octulosonic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CMP-KDO; CMP-2-KETO-3-DEOXY-OCTULOSONIC ACID; AC1L9IPX; CMP-3-deoxy-D-manno-octulosonate; CMP-beta-KDO; cytidine 5'-monophosphate 3-deoxy-beta-d-gulo-oct-2-ulo-pyranosonic acid; C04121; CHEBI:86284; DB04482; CMP-3-deoxy-beta-D-manno-octulosonic acid; cytidine 5'-[(3-deoxy-beta-D-manno-oct-2-ulopyranosonyl) hydrogen phosphate]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
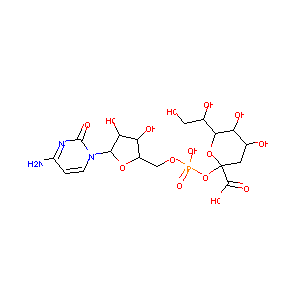 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 543.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -6.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
