Details of the Drug
General Information of Drug (ID: DMMBYGE)
| Drug Name |
Benzenesulfonyl
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
benzenesulfinate; 16722-50-2; BENZENESULFONYL; phenylsulfinate; phenyl sulfinate; Phenylsulfinsaure; (Phenylsulfonyl)radical; AC1MYJCD; Benzenethiol S,S-dioxide; C6H5O2S; SCHEMBL13707414; CHEBI:38100; MolPort-019-857-533; JEHKKBHWRAXMCH-UHFFFAOYSA-M; STL483233; HTS027703; ZINC150345494; AKOS015890258; AB00990983-01
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
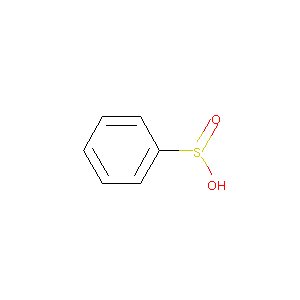 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 141.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
