Details of the Drug
General Information of Drug (ID: DMMC0I7)
| Drug Name |
METHIOTHEPIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
methiothepin; Metitepine; Methiothepine; Metitepinum; Metitepina; Metitepine [INN]; Metitepinum [INN-Latin]; Metitepina [INN-Spanish]; BRN 0626221; 20229-30-5; (+-)-10-(4-Methylpiperazinyl)-8-(methylthio)-10,11-dihydrodibenzo(b,f)thiepin; (+-)-8-Methylthio-10-(4-methylpiperazino)-10,11-dihydrodibenzo(b,f)thiepin; (+-)-1-(10,11-Dihydro-8-(methylthio)dibenzo(b,f)thiepin-10-yl)-4-methylpiperazine; CHEBI:64203; 1-(10,11-Dihydro-8-(methylthio)dibenzo(b,f)thiepin-10-yl)-4-methylpiperazine; NCGC00024665-03; DSSTox_CID_24000
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
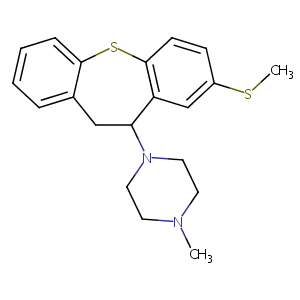 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 356.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
