Details of the Drug
General Information of Drug (ID: DMN7EUO)
| Drug Name |
N6022
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N6022; 1208315-24-5; N-6022; UNII-80LIU5P95D; 80LIU5P95D; CHEMBL1738699; 3-(5-(4-(1H-Imidazol-1-yl)phenyl)-1-(4-carbamoyl-2-methylphenyl)-1H-pyrrol-2-yl)propanoic acid; 3-{1-(4-Carbamoyl-2-Methylphenyl)-5-[4-(1h-Imidazol-1-Yl)phenyl]-1h-Pyrrol-2-Yl}propanoic Acid; N 6022; YVPGZQLRPAGKLA-UHFFFAOYSA-N; 3-[1-(4-carbamoyl-2-methylphenyl)-5-[4-(1H-imidazol-1-yl)phenyl]-1H-pyrrol-2-yl]propanoic acid; SCHEMBL244480; EX-A343; MolPort-035-789-691; HMS3653J15; BCP09367; s7589; ZINC66156654; BDBM50354475; 2573AH; AKOS030526322; DB12206
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
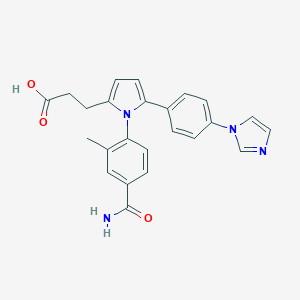 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 414.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 12 Disease of the respiratory system | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: CA23 Asthma | |||||||||||||||||||||||
| The Studied Tissue | Nasal and bronchial airway | |||||||||||||||||||||||
| The Studied Disease | Asthma [ICD-11:CA23] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
