| Drug Name |
Methoxyphenamine
|
| Synonyms |
Euspirol; METHOXYPHENAMINE; Methamphetamine, 2-methoxy; Methoxifenaminio; Methoxiphenadrin; Methoxiphenadrinum; Methoxyphenadrine; Methoxyphenamin; Methoxyphenamine [INN:BAN]; Methoxyphenaminum; Methoxyphenaminum [INN-Latin]; Metossifenamina [DCIT]; Metoxifenaminio [INN-Spanish]; Orthoxine; Ortodrinex; Ortoxine; Oxalacetic acid orthoxine; Proasma; 2-Methoxy-N,alpha-dimethylphenethylamin; 2-Methoxy-N-methylamphetamine; 2-Methoxymethamphetamine; 93-30-1; Asmi; EINECS 202-237-7; o-Methoxy-N,alpha-dimethylphenethylamine
|
| ATC Code |
- R03CB02: Methoxyphenamine
- R03CB: Non-selective beta-adrenoreceptor agonists
- R03C: ADRENERGICS FOR SYSTEMIC USE
- R03: DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R: RESPIRATORY SYSTEM
|
| Structure |
|
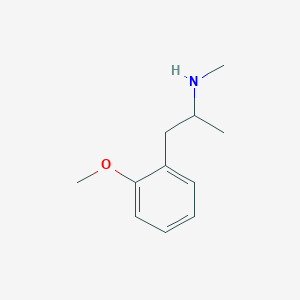 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
179.26 |
|
| Logarithm of the Partition Coefficient (xlogp) |
2.6 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
2 |
| Chemical Identifiers |
- Formula
- C11H17NO
- IUPAC Name
1-(2-methoxyphenyl)-N-methylpropan-2-amine - Canonical SMILES
-
CC(CC1=CC=CC=C1OC)NC
- InChI
-
OEHAYUOVELTAPG-UHFFFAOYSA-N
- InChIKey
-
1S/C11H17NO/c1-9(12-2)8-10-6-4-5-7-11(10)13-3/h4-7,9,12H,8H2,1-3H3
|
| Cross-matching ID |
- PubChem CID
- 4117
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR1049
|
|
|
|
|
|
|
|

