Details of the Drug
General Information of Drug (ID: DMNAKLY)
| Drug Name |
Remikiren
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
REM; Remikiren [INN]; Remikiren (INN); Ro 42-5892; Ro-42-5892; (2R)-2-benzyl-3-tert-butylsulfonyl-N-[(2S)-1-[[(2R,3S,4R)-1-cyclohexyl-4-cyclopropyl-3,4-dihydroxybutan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]propanamide; (2S)-2-benzyl-3-tert-butylsulfonyl-N-[(2S)-1-[[(2S,3R,4S)-1-cyclohexyl-4-cyclopropyl-3,4-dihydroxybutan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]propanamide; (S)-alpha-((S)-alpha-((t-butylsulfonyl)methyl)hydrocinnamamido)-N-((1S,2R,3S)-1-(cyclohexylmethyl)-3-cyclopropyl-2,3-dihydroxypropyl)imidazole-4-propionamide; (alphaS)-alpha-((alphaS)-alpha-((tert-Butylsulfonyl)methyl)hydrocinnamamido)-N-((1S,2R,3S)-1-(cyclohexylmethyl)-3-cyclopropyl-2,3-dihydroxypropyl)imidazole-4-propionamide; 2-benzyl-3-tert-butylsulfonyl-N-[1-[(1-cyclohexyl-4-cyclopropyl-3,4-dihydroxybutan-2-yl)amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]propanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
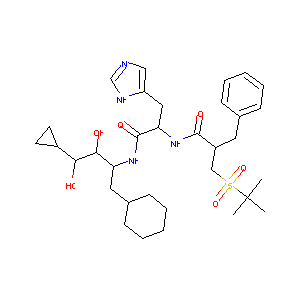 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 630.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
