| Drug Name |
Medrogestone
|
| Synonyms |
Colpro; Colprone; Etogyn; MEDROGESTONE; Medrogesterone; Medrogeston; Medrogestona; Medrogestona [INN-Spanish]; Medrogestone (USAN/INN); Medrogestone [USAN:INN:BAN]; Medrogestonum; Medrogestonum [INN-Latin]; Metrogestone; Prothil; R 13615; SCHEMBL140614; 077DN93G5B; 6,17-Dimethyl-6-dehydroprogesterone; 6,17-Dimethylpregna-4,6-diene-3,20-dione; 6-Methyl-6-dehydro-17-methylprogesterone; 977-79-7; AY 13615S; AY-62022; BRN 2302887; EINECS 213-555-0; NSC 123018; NSC-123018; Pregna-4,6-diene-3,20-dione, 6,17-dimethyl-; UNII-077DN93G5B
|
| Affected Organisms |
Humans and other mammals
|
| ATC Code |
- G03DB03: Medrogestone
- G03DB: Pregnadien derivatives
- G03D: PROGESTOGENS
- G03: SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM
- G: GENITO URINARY SYSTEM AND SEX HORMONES
|
| Structure |
|
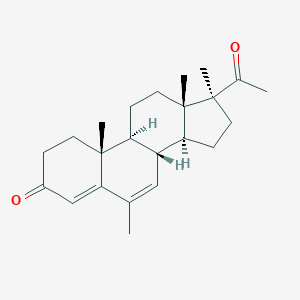 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
340.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.1 |
| Rotatable Bond Count (rotbonds) |
1 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
2 |
| ADMET Property |
- Absorption Tmax
-
The time to maximum plasma concentration (Tmax) is 2 h
[1]
- Half-life
-
The concentration or amount of drug in body reduced by one-half in 4 hours
[2]
|
| Chemical Identifiers |
- Formula
- C23H32O2
- IUPAC Name
(8R,9S,10R,13S,14S,17S)-17-acetyl-6,10,13,17-tetramethyl-2,8,9,11,12,14,15,16-octahydro-1H-cyclopenta[a]phenanthren-3-one - Canonical SMILES
-
CC1=CC2C(CCC3(C2CCC3(C)C(=O)C)C)C4(C1=CC(=O)CC4)C
- InChI
-
HCFSGRMEEXUOSS-JXEXPEPMSA-N
- InChIKey
-
1S/C23H32O2/c1-14-12-17-18(21(3)9-6-16(25)13-20(14)21)7-11-23(5)19(17)8-10-22(23,4)15(2)24/h12-13,17-19H,6-11H2,1-5H3/t17-,18+,19+,21-,22-,23+/m1/s1
|
| Cross-matching ID |
- PubChem CID
- 9949848
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR1010
|
|
|
|
|
|
|
|

