| Drug Name |
Methamphetamine hydrochloride
|
| Synonyms |
Methamphetamine HCL; Methamphetamine hydrochloride; Methamphetaminium chloride; Methylamphetamine hydrochloride; Methylisomyn; Norodin hydrochloride; Adipex; Chestox; Deofed; Des-O-E; Desepin; Desodex; Desoxo-5; Desoxyfed; Desoxyne; Destim; Dexoval; Dexstim; Dextim; Dosoxy; Doxyfed; Drinalfa; Efroxine; Eufodrinal; Gerovit; Hyphet; Isophen; Isophen (VAN); Obedrin-LA; Pervitin; Philopon; Soxysympamine; Syndrox; Tonedron; d-Methaphetamine hydrochloride; (+)-Methamphetamine hydrochloride; 51-57-0; S-(+)-Methamphetamine hydrochloride
|
| Structure |
|
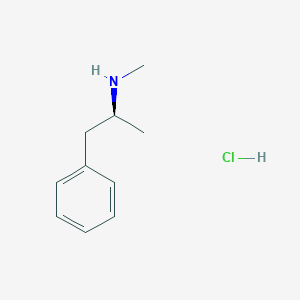 |
|
3D MOL is unavailable
|
2D MOL
|
#Ro5 Violations (Lipinski):
0 |
Molecular Weight |
185.69 |
| Logarithm of the Partition Coefficient |
Not Available |
| Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
| Hydrogen Bond Acceptor Count |
1 |
| Chemical Identifiers |
- Formula
- C10H16ClN
- IUPAC Name
(2S)-N-methyl-1-phenylpropan-2-amine;hydrochloride - Canonical SMILES
-
CC(CC1=CC=CC=C1)NC.Cl
- InChI
-
TWXDDNPPQUTEOV-FVGYRXGTSA-N
- InChIKey
-
1S/C10H15N.ClH/c1-9(11-2)8-10-6-4-3-5-7-10;/h3-7,9,11H,8H2,1-2H3;1H/t9-;/m0./s1
|
| Cross-matching ID |
- PubChem CID
- 66124
- ChEBI ID
-
- CAS Number
-
- INTEDE ID
- DR1041
|
|
|
|
|
|
|
|

