Details of the Drug
General Information of Drug (ID: DMO5SZX)
| Drug Name |
Diphenoxylate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Difenossilato; Difenoxilato; Diphenoxalate; Diphenoxylatum; Difenossilato [DCIT]; Difenoxilato [Spanish]; Diphenoxylate HCl; Diphenoxylate Monohydrochloride; Diphenoxylate hydrochloride; Diphenoxylatum [Latin]; NIH 7562; R 1132; Difenoxilato [INN-Spanish]; Diphenoxylate (INN); Diphenoxylate [INN:BAN]; Diphenoxylate hydrochloride (USP); Diphenoxylate hydrochloride(usp); Diphenoxylatum [INN-Latin]; R 1132 (antiperistaltic); R-1132; Co-phenotrope (TN); Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecotate; Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecotate monohydrochloride; Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylate; Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidine-4-carboxylate; Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidine-4-carboxylate hydrochloride; Isonipecotic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester; Ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidin-1-ium-4-carboxylate chloride; Isonipecotic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester, hydrochloride; 1-(3-Cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylic acid ethyl ester; 1-(3-Cyano-3,3-diphenylpropyl)-4-phenyl-isonipecotic acid ethyl ester; 1-(3-Cyano-3,3-diphenylpropyl)-4-phenylisonipecotic acid ethyl ester hydrochloride; 1-(3-cyano-3,3-diphenylpropyl)-4-(ethoxycarbonyl)-4-phenylpiperidinium chloride; 2,2-Diphenyl-4-(4-carbethoxy-4-phenylpiperidino)butyronitrile; 4-Ethoxycarbonyl-alpha,alpha,4-triphenyl-1-piperidinebutyronitrile, hydrochloride; 4-Piperidinecarboxylic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester; 4-Piperidinecarboxylic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester (9CI); 4-Piperidinecarboxylic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester, monohydrochloride; 4-ethoxycarbonyl-alpha,alpha,4-triphenyl-1-piperidinebutyronitrile hydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
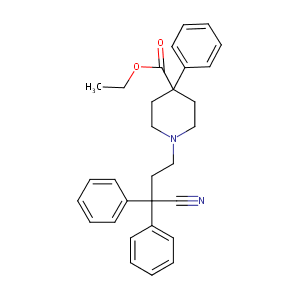 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 452.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Diarrhea | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ME05.1 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Diphenoxylate (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
References
