Details of the Drug
General Information of Drug (ID: DMO87YS)
| Drug Name |
GSK065
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL4091152; (R)-3-(5-Chloro-6-(1-(pyridin-2-yl)ethoxy)benzo[d]isoxazol-3-yl)propanoic acid; 3-[5-Chloranyl-6-[(1~{r})-1-Pyridin-2-Ylethoxy]-1,2-Benzoxazol-3-Yl]propanoic Acid; 1953156-61-0; 8R5; SCHEMBL17844622; GTPL10358; BDBM50266003; GSK3335065; inhibitor C1 [PMID: 28604669]; ClC=1C(=CC2=C(C(=NO2)CCC(=O)O)C=1)O[C@H](C)C1=NC=CC=C1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
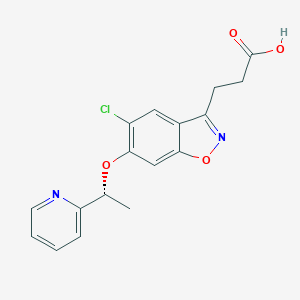 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 346.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
