Details of the Drug
General Information of Drug (ID: DMOMLHJ)
| Drug Name |
Lymecycline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aureociclin; Aureomicina; Aurofac; Biovetin; Centraureo; Chlortetracyclin; Chrusomykine; Limeciclina; Lymecyclinum; Orospray; Acronize PD; Aurofac 10; Tetrlysal 300; Lymecycline (INN); Tetralysal 300 (TN); N(2)-(((+)-5-Amino-5-carboxypentylamino)methyl)tetracycline; N(6)-[({[(4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracen-2-yl]carbonyl}amino)methyl]-L-lysine; (+)-N-(5-Amino-5-carboxypentylaminomethyl)-4-dimethylamino-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxonaphthacene-2-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
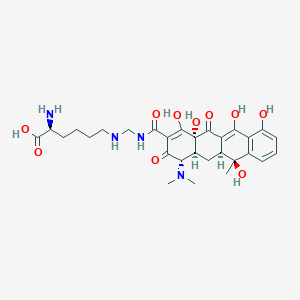 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 602.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
