Details of the Drug
General Information of Drug (ID: DMORSGU)
| Drug Name |
Cefodizime
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
AC1NSFIB; CDZM; CHEBI:63214; CHEMBL2303613; Cefodizima; Cefodizima [INN-Spanish]; Cefodizime; Cefodizime (INN); Cefodizime Acid; Cefodizime [INN:BAN]; Cefodizimum; Cefodizimum [INN-Latin]; Cefodizme; DTXSID2022757; Diezime; EC 700-301-3; EX-A1379; HR 221 [AS SODIUM]; HR-221; HR-221 [As Sodium]; J-700161; Modivid; Neucef; S-771221B [As Sodium]; SCHEMBL151101; THR 221 [AS SODIUM]; THR-221; THR-221 [As Sodium]; Timecef; UNII-Z31298J4HQ; Z31298J4HQ
|
|||||
| Therapeutic Class |
Anti-Bacterial Agents
|
|||||
| ATC Code | ||||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
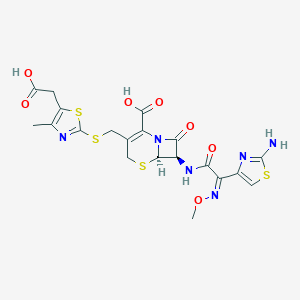 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 584.655 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 0.2 | |||||
| Rotatable Bond Count (rotbonds) | 10 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
