Details of the Drug
General Information of Drug (ID: DMP2DNS)
| Drug Name |
Rambazole
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Talarozole; 201410-53-9; R-115866; N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)benzo[d]thiazol-2-amine; R115866; CHEMBL459505; C21H23N5S; Rambazole (TN); Talarozole (USAN/INN); Talarozole [USAN:INN]; R 115866; N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)-2-benzothiazolamine; Talarozole pound> SCHEMBL721201; CHEBI:102167; MolPort-018-666-712; SNFYYXUGUBUECJ-UHFFFAOYSA-N; BCP28256; BCP21218; BDBM50253810; 0328AB; AKOS005067289; DB13083; CS-1343; NCGC00378894-01; HY-14531; AX8224298; D09385; W-5674; MEN13510
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
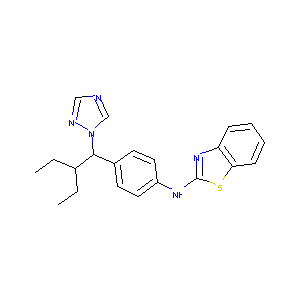 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 377.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Psoriasis vulgaris | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EA90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
