Details of the Drug
General Information of Drug (ID: DMP2G5W)
| Drug Name |
(3-amino-5-bromobenzofuran-2-yl)(phenyl)methanone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL591458; (3-Amino-5-bromo-benzofuran-2-yl)-phenyl-methanone; (3-amino-5-bromo-1-benzofuran-2-yl)(phenyl)methanone; SMR000010549; MLS000031617; AC1LCKCT; regid849115; Oprea1_732422; Oprea1_454471; MLS002534670; (3-amino-5-bromobenzofuran-2-yl)(phenyl)methanone; SCHEMBL13102601; ZINC97827; CTK5I8073; MolPort-000-164-762; HMS2395L21; STK078344; BDBM50306745; AKOS000510990; MCULE-9438936220; ST037109; BAS 01507283; EU-0018517; 3-amino-5-bromobenzo[d]furan-2-yl phenyl ketone; SR-01000523207; SR-01000523207-1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
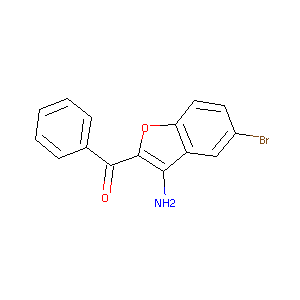 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 316.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
