Details of the Drug
General Information of Drug (ID: DMP3CSV)
| Drug Name |
AZD4604
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JAK1-IN-7; AZD4604; 2241039-81-4; AZD-4604; JAC34RRR7S; (2R)-N-[3-[5-fluoro-2-(2-fluoro-3-methylsulfonylanilino)pyrimidin-4-yl]-1H-indol-7-yl]-3-methoxy-2-(4-methylpiperazin-1-yl)propanamide; (R)-N-(3-(5-Fluoro-2-((2-fluoro-3-(methylsulfonyl)phenyl)amino)pyrimidin-4-yl)-1H-indol-7-yl)-3-methoxy-2-(4-methylpiperazin-1-yl)propanamide; londamocitinib; UNII-JAC34RRR7S; CHEMBL4447181; SCHEMBL20399395; GTPL11716; BDBM488779; EX-A5343; US10961228, Example 35; AKOS040733485; AT39356; example 35 [WO2018134213A1]; MS-30607; HY-126294; CS-0101485; (R)-N-(3-(5-fluoro-2- (2-fluoro-3- (methylsulfonyl)phenyl amino)pyrimidin-4-yl)- 1H-indol-7-yl)-3- methoxy-2-(4- methylpiperazin-1- yl)propanamide; 1-Piperazineacetamide, N-(3-(5-fluoro-2-((2-fluoro-3-(methylsulfonyl)phenyl)amino)-4-pyrimidinyl)-1H-indol-7-yl)-alpha-(methoxymethyl)-4-methyl-, (alphaR)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
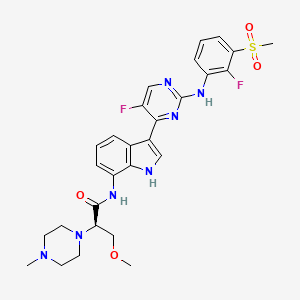 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
