Details of the Drug
General Information of Drug (ID: DMP83UZ)
| Drug Name |
Fumaric acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fum; Fumarsaeure; Acidum fumaricum; Allomaleic acid; Allomalenic acid; Ammonium fumarate; Boletic acid; Butenedioic acid; Kyselina fumarova; Kyselina fumarova [Czech]; Lichenic acid; Tumaric acid; F0067; OR17920; FC 33 (acid); Fumarate, 10; Fumaric acid (8CI); Fumaric acid (NF); Lichenic acid (VAN); S04-0167; Trans-Butenedioic acid; U-1149; E-2-Butenedioic acid; Trans-2-Butenedioic acid; USAF EK-P-583; Trans-1,2-Ethylenedicarboxylic acid; Trans-but-2-enedioic acid; (2E)-2-butenedioic acid; (2E)-but-2-enedioic acid; (E)-2-Butenedioic acid; 1,2-Ethylenedicarboxylic acid, (E); 2-(E)-Butenedioic acid; 2-Butenedioic acid; 2-Butenedioic acid (2E-(9CI)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
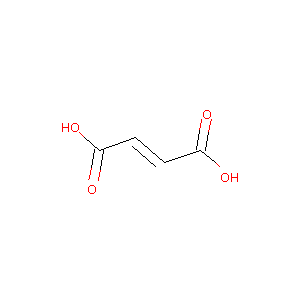 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 116.07 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
