Details of the Drug
General Information of Drug (ID: DMPC4EK)
| Drug Name |
EP-217609
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Neutralizable NAPAP analog (thrombosis), Endotis; Neutralizable NAPAP analog (thrombosis), Organon; Fondaparinux analog + thrombin inhibitor (thrombosis), Endotis; Neutralizable Factor IIa/Factor X dual inhibitor (thrombosis), Endotis; Neutralizable Factor IIa/Factor X dual inhibitor (thrombosis), Organon
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
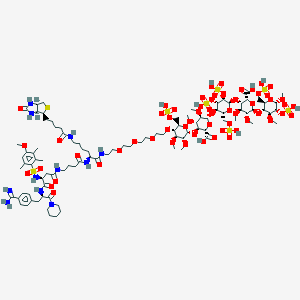 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 2647.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -7.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 74 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 18 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 62 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
