Details of the Drug
General Information of Drug (ID: DMPDO49)
| Drug Name |
PSILOCIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PSILOCIN; 4-Hydroxy-N,N-dimethyltryptamine; Psilocine; Psilotsin; 520-53-6; Psilocyn; 3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol; 3-(2-(Dimethylamino)ethyl)indol-4-ol; UNII-CMS88KUW0G; N,N-Dimethyl-4-Hydroxytryptamine; CX-59; EINECS 208-296-5; CMS88KUW0G; BRN 0160503; CHEMBL65547; CHEBI:8613; DEA No. 7438; 3-(2-(Dimethylamino)ethyl)-1H-indol-4-ol; NCGC00247728-01; P-7800; 1H-Indol-4-ol, 3-[2-(dimethylamino)ethyl]-; INDOL-4-OL, 3-(2-(DIMETHYLAMINO)ETHYL)-; Indol-4-ol, 3-[2-(dimethylamino)ethyl]-; Psilocin solution; AC1L1JCI; 4-OH-DMT
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
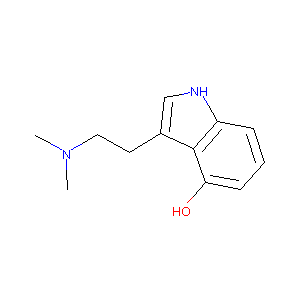 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 204.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
