Details of the Drug
General Information of Drug (ID: DMPH916)
| Drug Name |
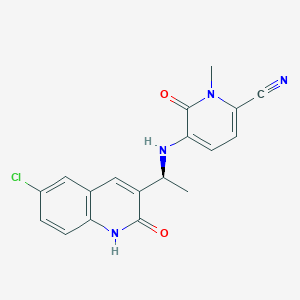
Olutasidenib
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
FT-2102; NEQYWYXGTJDAKR-JTQLQIEISA-N; Olutasidenib; UNII-0T4IMT8S5Z; 0T4IMT8S5Z; (S)-5-((1-(6-chloro-2-oxo-1,2-dihydroquinolin-3-yl)ethyl)amino)-1-methyl-6-oxo-1,6-dihydropyridine-2-carbonitrile; Olutasidenib [USAN]; SCHEMBL17603134; HY-114226; CS-0080183; 5-(((1S)-1-(6-chloro-2-oxo-1,2-dihydroquinolin-3-yl)ethyl)amino)-1-methyl-6-oxo-1,6-dihydropyridine-2-carbonitrile; 2-Pyridinecarbonitrile, 5-(((1S)-1-(6-chloro-1,2-dihydro-2-oxo-3-quinolinyl)ethyl)amino)-1,6-dihydro-1-methyl-6-oxo-; 1887014-12-1
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
