Details of the Drug
General Information of Drug (ID: DMPML15)
| Drug Name |
RAZAXABAN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Razaxaban Hydrochloride; UNII-7CLJ1MEZ8V; BMS-561389; 7CLJ1MEZ8V; 405940-76-3; DPC 906; Razaxaban Hydrochloride [USAN]; Razaxaban hydrochloride (USAN); CHEMBL558198; DPC-906; BMS 561389; Razaxaban HCl; AC1L4BWY; SCHEMBL6885838; DTXSID10193598; Razaxaban hydrochloride, > D04029; 1H-Pyrazole-5-carboxamide, 1-(3-amino-1,2-benzisoxazol-5-yl)-N-(4-(2-((dimethylamino)methyl)-1H-imidazol-1-yl)-2-fluorophenyl)-3-(trifluoromethyl)-, monohydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
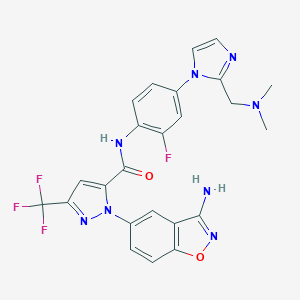 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 528.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
