Details of the Drug
General Information of Drug (ID: DMPT74U)
| Drug Name |
Gramicidin D
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Gramicidins; Gramoderm; Gramacidine S; Gramicidin C; Gramicidin Dubos; Gramicidin J; Gramicidin K; Gramicidin P; Gramicidin S; Gramicidin S [INN]; Gramicidina D; Gramicidina S; Gramicidinum S; Neosporin Ophthalmic Solution; Gramicin S 1; Gramacidine S [INN-French]; Gramicidin A(1); Gramicidin [INN:BAN]; Gramicidina S [INN-Spanish]; Gramicidina [INN-Spanish]; Gramicidine [INN-French]; Gramicidinum S [INN-Latin]; Gramicidinum [INN-Latin]; Gramicin S-A; Cyclo(L-valyl-L-ornithyl-L-leucyl-D-phenylalanyl-L-prolyl-L-valyl-L-ornithyl-L-leucyl-D-phenylalanyl-L-prolyl); 1,10-anhydro(L-leucyl-D-phenylalanyl-L-prolyl-L-valyl-L-ornithyl-L-leucyl-D-phenylalanyl-L-prolyl-L-valyl-L-ornithine)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||
| Affected Organisms |
Staphylococcus aureusStreptococcus agalactiaeStreptococcus pneumoniaeEscherichia coliHaemophilus influenzaeKlebsiellaEnterobacterNeisseria gonorrhoeaeNeisseria meningitidisPseudomonas aeruginosa
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
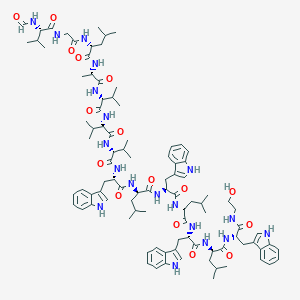 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1811.2 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 10.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 50 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 20 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 16 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
