Details of the Drug
General Information of Drug (ID: DMPU7KE)
| Drug Name |
(4-Methyl-2-oxo-2H-quinolin-1-yl)-acetic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
103368-21-4; (4-Methyl-2-oxo-2H-quinolin-1-yl)-acetic acid; 1(2h)-quinolineacetic acid, 4-methyl-2-oxo-; NSC108456; (4-methyl-2-oxoquinolin-1(2H)-yl)acetic acid; CHEMBL64455; 2-(4-methyl-2-oxoquinolin-1(2H)-yl)acetic acid; BAS 02839786; (4-Methyl-2-oxo-1(2H)-quinolinyl)acetic acid; 2-(4-methyl-2-oxohydroquinolyl)acetic acid; AC1L6JZ1; AC1Q6I1O; MLS000714792; SCHEMBL15709048; CTK4A2028; DTXSID20296246; MolPort-001-984-878; ZINC380285; HMS2670L06; HMS1681E20; HMS3482A22; ZX-AN005388; ALBB-005471; BB_SC-06267; ZX-CM004260; BBL010328
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
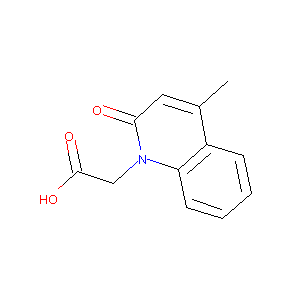 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 217.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
