Details of the Drug
General Information of Drug (ID: DMPYQ7B)
| Drug Name |
Trimebutine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Trimebutine maleate; Trimedat; Dromostat; Ibutin; Digerent polifarma; EINECS 251-845-9; TM 906; (1-Ethyl-1-phenyl-2-(3,4,5-trimethoxybenzoyloxy)ethyl)dimethylammonium hydrogen maleate; AC1O5HFA; Benzoic acid, 3,4,5-trimethoxy-, beta-(dimethylamino)-beta-ethylphenethyl ester, maleate (1:1); 34140-59-5; SCHEMBL1652810; C22H29NO5.C4H4O4; AKOS026750056; LS-38470; dimethyl-[2-phenyl-1-(3,4,5-trimethoxybenzoyl)oxybutan-2-yl]azanium; (E)-4-hydroxy-4-oxobut-2-enoate; Benzoic acid, 3,4,5-trimethoxy-, beta-(dimethylamino)-beta-ethyl
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
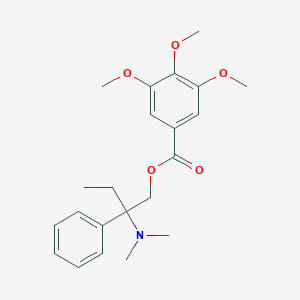 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 387.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
