Details of the Drug
General Information of Drug (ID: DMQ5ZVK)
| Drug Name |
JNJ-54175446
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CWFVVQFVGMFTBD-SECBINFHSA-N; UNII-32524GLF40; 32524GLF40; 1627902-21-9; SCHEMBL16036477; BDBM254266; AKOS032947046; AS-35269; J3.655.327H; US9464084, 158; (R)-(2-chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyrimidin-2-yl)-4-methyl-6,7-dihydro-1H-[1,2,3]triazolo[4,5-c]pyridin-5(4H)-yl)methanone; (r)-(2-chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyrimidin-2-yl)-4-methyl-1,4,6,7-tetrahydro-5h-[1,2,3]triazolo[4,5-c]pyridin-5-yl)methanone; Methanone, (2-chloro-3-(trifluoromethyl)phenyl)((4R)-1-(5-fluoro-2-py
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
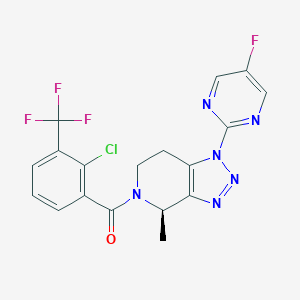 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 440.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Major depressive disorder | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70.3 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
