Details of the Drug
General Information of Drug (ID: DMQ6W4I)
| Drug Name |
Substance P
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Substance P; 33507-63-0; Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2; CCRIS 7229; UNII-675VGV5J1D; EINECS 251-545-8; CHEMBL235363; 675VGV5J1D; 12769-48-1; P substance; Neurokinin P; Substanz-P; C63H98N18O13S; Substance P analogue; AC1Q5IQE; AC1L1VX3; tachykinin substance P (SP); SCHEMBL1116347; GTPL2098; ArgProLysProGlnGlnPhePheGlyLeuMet; MolPort-023-276-010; ADNPLDHMAVUMIW-CUZNLEPHSA-N; 2053AH; BDBM50001450; AKOS024456424; NCGC00167123-01; 11035-08-8; LS-147256; Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Sequence |
>DE = Substance P
RPKPQQFFGLM |
||||||||||||||||||||||
| Structure |
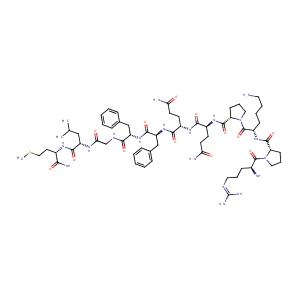 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1347.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 42 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 15 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 17 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
