Details of the Drug
General Information of Drug (ID: DMQ704Z)
| Drug Name |
Quinazoline derivative 16
|
|||||
|---|---|---|---|---|---|---|
| Synonyms | PMID27646564-Compound-12-2 | |||||
| Drug Type |
Small molecular drug
|
|||||
| Structure | 3D Structure is Not Available |
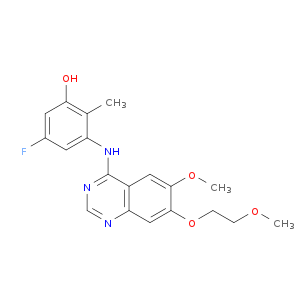 |
||||
| 3D MOL | 2D MOL | |||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
