Details of the Drug
General Information of Drug (ID: DMQHJTF)
| Drug Name |
Green tea
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Oxotremorine; Oxytremorine; Oxotremorin; Tremorine, oxo-; 70-22-4; 2'-Oxopyrrolidino-1-pyrrolidino-4-butyne; MLS000766260; UNII-5RY0UWH1JL; EINECS 200-728-0; 5RY0UWH1JL; NSC 330497; 1-(4-pyrrolidin-1-ylbut-2-ynyl)pyrrolidin-2-one; BRN 1530948; CHEMBL7634; 1-(4-(Pyrrolidin-1-yl)but-2-ynyl)pyrrolidin-2-one; CHEBI:7851; 2-PYRROLIDINONE, 1-(4-(1-PYRROLIDINYL)-2-BUTYNYL)-; RSDOPYMFZBJHRL-UHFFFAOYSA-N; 2-Pyrrolidinone, 1-[4-(1-pyrrolidinyl)-2-butynyl]-; Tocris-0843; Spectrum_001875; Spectrum_001448; AC1L1ILJ; Spectrum5_001099
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
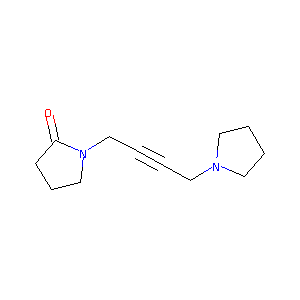 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 206.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
